Properties of Lubricants:-
Lubricants have several properties, some important properties are
given below:-
1.
Viscosity:
It is the property of a liquid or a fluid by virtue of which it offers
resistance to its own flow. If two layers of a liquid separated by a distance’s
and moving with a relative velocity difference ‘v’ then force per unit area(f)
required to maintain this velocity difference is given by-
f=nv/d
Where
n is the coefficient of viscosity.Viscosity
is the most important property of any lubricating oil. If the viscosity of the
oil is too low a liquid oil film cannot be maintained between two moving
surfaces and excessive wearing takes place.
Determination of Viscosity:
The
apparatus which is used to determine the viscosity is known as viscometer. In
industry viscosity of lubricating oil is determined by Redwood, say bolt and
Angler instrument. In the Redwood viscometer the measure of viscosity of oil is
the time in seconds for 0ml of oil to flow through standard orifice under a
given set of condition.
By
Redwood Viscometer: It is of two
types:- (i). Rw1 , (ii).Rw2
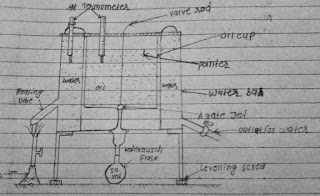
Fig.1.(a). Redwood viscometer no. 1
Working:
The
apparatus is leveled and water bath is filled with water. A thermometer is
placed in water bath. The oil cup is cleaned and ball of value rod is places on
the agate jet to close it. An empty cleaned kohlrausch flask is kept just below
the jet. The experimental oil is listed in oil cup up to a pointer. A
thermometer is also placed in oil cup read the temperature of oil. Now water
bath is heated up to a certain temperature with constant stirring the water.
When the oil at desired temperature heating is stopped and the ball valve is
fitted and suspended from thermometer bracket. The time taken for 50ml of the
oil to pass through the agate jet and collected into kohlrausch mask is noted.
Now the valve immediately closed to prevent any overflow of the oil. The
experiment is repeated and the mean value of time of flow for 50ml oil sample
is reported as a result expressed in “Redwood No. 1 seconds” at a particular
temperature then
n1/n2 =r1t1/r2t2
where
n1= viscosity of experimental oil sample
n2=
viscosity of standard liquid
r1=
Density of experimental liquid
t1=
Time of flow of experimental oil sample
Thus
the value of n1 i.e., viscosity of experimental oil is calculated in
‘poise’.
2.
Viscosity
Index:-
The
arbitrary scale which measures the variation of viscosity with temperature is
called viscosity index. Generally the viscosity of an oil decreases with rise
in temperature. The viscosity of a good lubricant should not change very much
with the rise in temperature. If the viscosity of oil is very much affected
with the rise in temperature it is called low viscosity index. Similarly if the
viscosity of oil is slightly affected with the rise in temperature, it is
called high viscosity index lubricant. A good lubricant should have high
viscosity index.
Determination of Viscosity
index:
For
the determination of viscosity index of experimental oil the viscosities of
testing oil at 100ºf
and 210ºf
are found out. The viscosity at 100ºf
of the oil under test is represented by ‘U’. Now we compare the viscosity of
oil under test with two standard oil, one with the highest viscosity index
(VI=100) and another with the lower viscosity index(VI=0). Pennsylvanian oils
have highest viscosity index(VI=100) and Gulf oil have lowest viscosity index(VI=0). Now viscosity index (VI) may be
calculated as:-
Viscosity
Index(VI)= L-U/L-H*100
Where
‘U’ is the viscosity of experimental oil at 100ºf.
And ‘L’ is the viscosity of low viscosity index standard oil (Gulf oil having
VI=0 ) at 100ºf
and also having the same viscosity of experimental oil at 210ºf. And ‘H’ viscosity of high
index standard oil (Pennsylvanian oil having VI=100) at 100ºf and also having the same
viscosity of experimental oil at 210ºf.
Viscosity curves:-
 |
| Fig.2.(a). |
3.
Cloud-Point
and Pour Point:-
When
an oil is cooled slowly, the temperature at which an oil becomes cloudly in
appearance it is called its ‘cloud point’. The temperature at which the oil
ceases to flow or pour is called ‘pour point’. Cloud point and pour point
indicates the suitability of lubricants in cold conditions.
Determination of Cloud-point
and Pour-point:
Fig.3.(a). Cloud Point and Pour Point
It
is determined with the help of pour point apparatus as shown in fig(a). It
consists of a flat-bottomed tube for taking lubricating oil. The tube is
enclosed by a air jacket. The air-jacket is surrounded by freezing mixture
(ice+Cacl2) contained in a jar. The flat tube is half filled with
experimental oil. A thermometer is placed in the oil. The oil gets start cooling and
the temperature decrease slowly. At an interval of fall in temperature
every 1ºC,
the tube is withdrawn from the air jacket for a moment and examined and then
replaced in ice-bath immediately. The temperature, at which cloudness is noted
is recorded as the cloud-point. After this cooling is continued and the test tube
is removed from the cooling bath after every 3ºC
fall of temperature and tilted to observe the flow or pour of oil. The
temperature at which oil does not flow in the test tube, even when kept
horizontal for 5 second, is recorded as the pour point.
Significant: Cloud
point and pour point tells us a minimum temp at which oil can be used as a
lubricant.
4.
Flash
and Fire point:-
- Flash point: It is the lowest temperature of lubricant at which the lubricant gives enough vapours which burn for a moment, when a flame is brought near it.
- Fire Point: It is the lowest temperature of lubricant at which the lubricant gives enough vapours which burn continuously for at east five second when a small flame is brought near to is.
Significance: Flash
and fire point tells us the maximum temperature at which a lubricant can be
used.
Determination of Flash and
Fire point: The Flash and fire point is determined by penskey-marten’s apparatus as shown in fig.4.(a).
Fig.4.(a). Penskey-Marten's flash point apparatus
First
of all clean and dry all the parts of the apparatus and experimental
lubricating oil is filled up and the mark in the oil cup. The apparatus is
heated with constant stirring at the rate of about 1-2 revolutions per second.
The heating of oil cup is adjusted in such a way that the temperature of the
oil rises at the rate of about 5ºc
per minute. At every 1ºc
rise in temperature test flame is introduced for the moment with the help of a
shutter. The temperature at which a distinct
flash appears inside the up is recorded as the flash point of
lubricating oil. The heating is continued further and the test flame introduced
as before. The temperature at which the experimental lubricating oil catches
fire at least 5 seconds is recorded as its fire-point.
5.
Aniline
Point:-
It
is defined as the minimum temp. at which the lubricating oil is just miscible
with equal volume of aniline.
Significance: Aniline
point of any lubricant is a measure of aromatic content.
Determination of Aniline
Point:
Fig.5.(a). Aniline point apparatus
The whole apparatus may be
represented as in fig. The apparatus is cleaned and dried at 100-110ºc . An equal volume of pure
and dried aniline and dried oil sample have been taken in the test tube. This
test tube is fitted with electrically operated glass rod stirrer and a
thermometer . The test tube is inserted into an outer air-jacket made of heat
resistance glass. The aniline and oil sample mixture is stirred to get a
homogeneous solution . For the sometimes we use hot bath and stirring is
continued. The jacket is then withdrawn from the hot bath and the temperature
is allowed to fall at a rate below 1ºc
per minutes. For this purpose we may use cold bath. The temperature, at which
the two phases just separate out, is reported as the aniline point of the
sample.




Comments
Post a Comment